News
Preferred Networks and PFDeNA launch joint research project to develop a deep learning-based system to detect 14 types of cancers with a small amount of blood
2018.10.29
Aim to bring to market by 2021 to extend healthy life expectancy with early cancer detection
Oct. 29, 2018, Tokyo Japan – Preferred Networks, Inc. and PFDeNA Inc. will start research and development to create a blood test system that utilizes deep learning technology to detect 14 types of cancers*1 in their early stages.
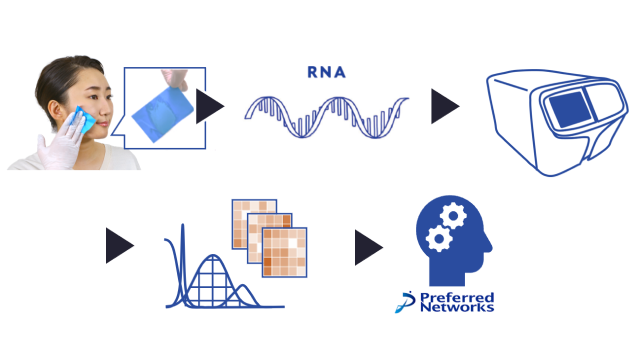
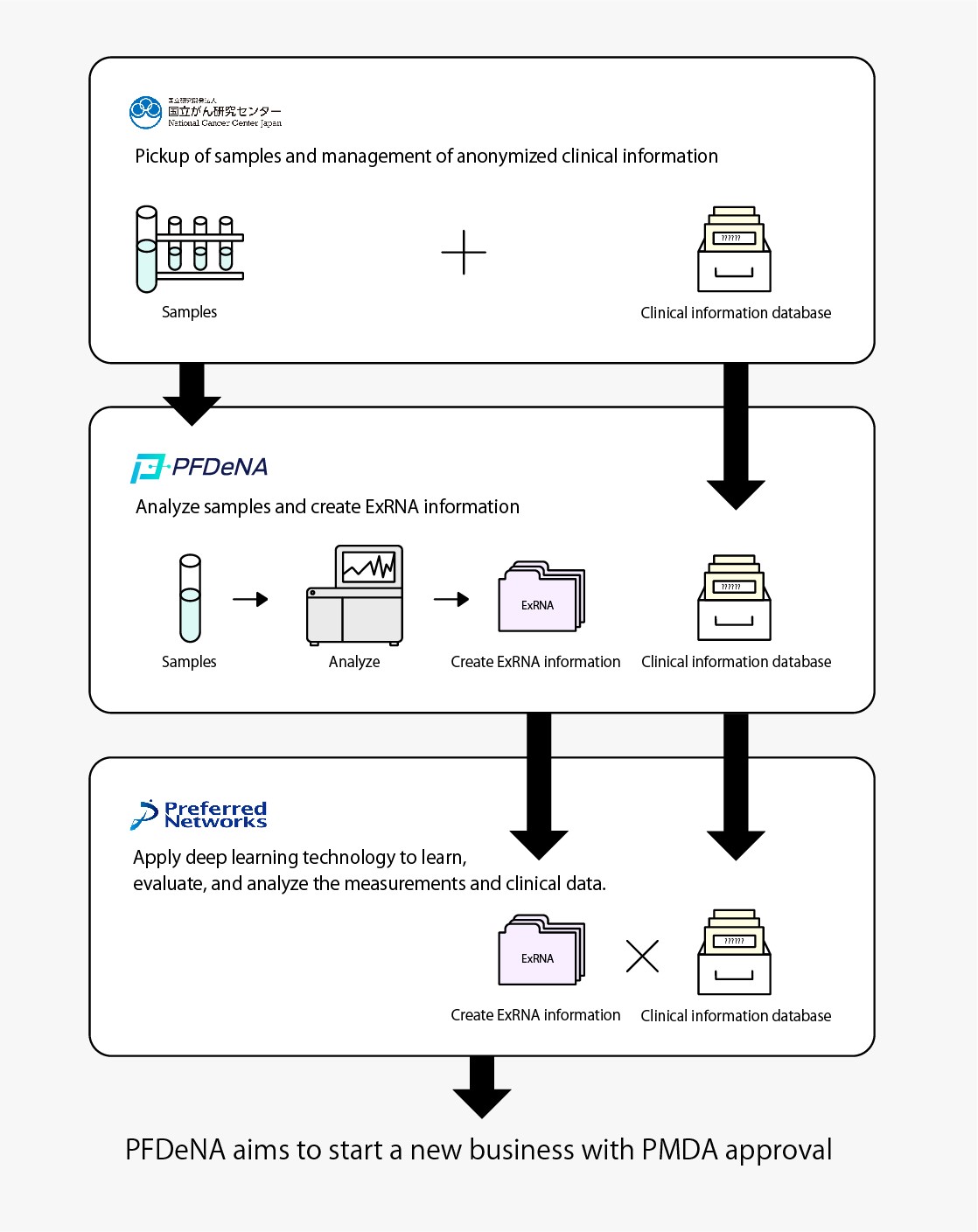
In this R&D initiative, PFN and PFDeNA will use blood samples (DNA repository samples) and clinical information, both collected by the National Cancer Center Japan (NCC) for research purposes with donor consent. PFDeNA will measure the expression levels of ExRNA*2 in the DNA repository samples by using a next-generation sequencer*3 in a manner that does not identify individuals. PFN will apply deep learning technology to learn, evaluate, and analyze the measurements together with clinical data. The aim is to put the resulting system to practical use, which will be able to accurately determine the presence or absence and kind of cancer based on ExRNA expression levels in the blood.
Social background
Cancer is the leading cause of death among Japanese people, with about one in two developing cancer in their lifetimes. The number of Japanese who died from cancer is more than 370,000 a year and continuing to rise. This amounts to one out of every 3.6 deaths being caused by cancer *.
Even though it is critical to detect cancer at an early stage, screening rates for various types of cancers remain at roughly 30%, one of the lowest among developed countries. Each type of cancer has its own screening methods and requires different areas and organs in our bodies to be tested. The level of accuracy differs from one test to another. The burden of taking these tests need to be reduced both physically and financially in order to improve the screening rates.
Against this backdrop, many studies have been reported recently on gene expression of ExRNAs which include miRNAs*4, bringing to light miRNA expressions that are unique biomarkers of cancer in each organ. Because the types or numbers of miRNAs expressed in bodily fluids will change once a person has cancer, researchers have high expectations that it will become easier to diagnose cancers using easily-collectible bodily fluids, such as blood.
Going forward
After PMDA’s*5 review and approval, PFN and PFDeNA aim to develop the results of this research into a business by 2021 and promote its widespread use in Japan.
The high-precision, low-impact screening system will require only a small amount of blood to detect 14 types of cancers in their early stages and is expected to become a common cancer test in the future. Through early cancer detection, PFN and PFDeNA will contribute to efforts to decrease the mortality rate, to reduce medical costs, to extending healthy life expectancy and increasing cancer screening rates in Japan.
*1 The 14 types of cancers covered in this research are stomach cancer, colon cancer, esophageal cancer, pancreatic cancer, liver cancer, bile duct cancer, lung cancer, breast cancer, ovarian cancer, cervical cancer, uterine cancer, prostate cancer, bladder cancer, and kidney cancer.
*2 ExRNA is an RNA existent in the blood and other bodily fluids, mainly miRNA (microRNA) in this research. miRNA helps regulate a variety of biological activities and is expected to be used as a diagnostic biomarker.
*3 A next-generation sequencer is a piece of equipment used to sequence the base pairs of human genes in parallel at high speed.
*4 miRNA is a ribonucleic acid that is about 20 bases long and plays a role in regulating gene expression.
*5 PMDA is an acronym for the Pharmaceuticals and Medical Devices Agency of Japan, which is an organization that conducts the scientific review for quality, efficacy, and safety of pharmaceuticals and medical equipment. https://www.pmda.go.jp/english/about-pmda/outline/0005.html
*Source: “Summary of Vital Statistics for 2017” (Ministry of Health, Labour and Welfare)